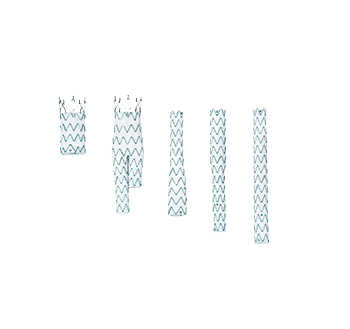
Configuration- Main body
Proximal graft diameter
Distal graft diameter
Total covered length
OD of delivery system
20mm/22mm
12mm
110mm
16Fr
24mm
12mm
110mm
18Fr
26mm/28mm
14mm
110mm
18Fr
30mm/32mm/34mm/36mm
14mm
110mm
20Fr
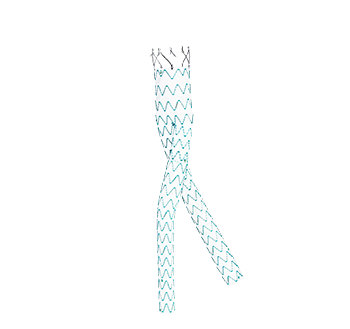
Configuration- Limb
Proximal graft diameter
Distal graft diameter
Total covered length
OD of delivery system
16mm
10mm/13mm/16mm
60mm/80mm/95mm/110mm/125mm/140mm/155mm
14Fr
16mm
16mm
155mm
16Fr
16mm
20mm
60mm/80mm/95mm/110mm/125mm/140mm/155mm
16Fr
16mm
24mm
60mm/80mm/95mm/110mm/125mm/140mm/155mm
18Fr
* exception- 16Fr, instead of 14Fr, applies to PAIL1616155
Configuration- Iliac extension
|
Proximal graft diameter |
Distal graft diameter |
Total covered length |
OD of delivery system |
|
10mm |
10mm |
80mm |
14Fr |
|
13mm |
13mm |
80mm |
14Fr |
|
20mm |
20mm |
80mm |
16Fr |
|
24mm |
24mm |
80mm |
18Fr |
Configuration- PAUI
Proximal graft diameter
Distal graft diameter
Total covered length
OD of delivery system
18mm/20mm/22mm
14mm
110mm
16Fr
24mm/26mm/28mm
14mm
110mm
18Fr
30mm/32mm/34mm/36mm
14mm
110mm
20Fr
Configuration- Cuff
Proximal graft diameter
Distal graft diameter
Total covered length
OD of delivery system
20mm/22mm
20mm/22mm
45mm/70mm
16Fr
24mm/26mm/28mm
24mm/26mm/28mm
45mm/70mm
18Fr
30mm/32mm/34mm/36mm
30mm/32mm/34mm/36mm
45mm/70mm
20Fr
* All of these specifications are for straight stent graft.
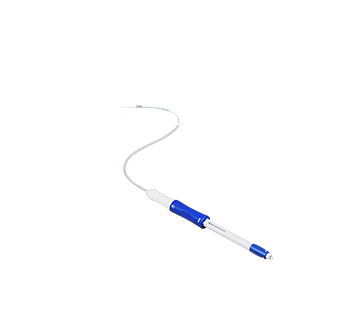
The clinical trial of Percutek's Abdominal Aortic Stent Graft System was an open-label, non-randomized, concurrent controlled clinical trial.
A total of 153 patients were enrolled in 11 sites, including 82 patients in the experimental group and 71 patients in the control group.
The primary effectiveness endpoint was the percentage of patients who were successfully treated for abdominal aneurysms. The success rate of was 95.8% in the experimental group and 90.6% in the control group.
Table 1 Success rate of abdominal aneurysm treatment (adjusted PPS set):
Experimental group (%, m/n)
Control group (%, m/n)
Success rate
96.0% (72/75)
91.0% (61/67)
The primary safety endpoint was the percentage of patients who had no major adverse events during the perioperative period. 97.5% of patients in the experimental group had no major clinical adverse events within 30 days (perioperative period), compared with 97.0% of patients in the control group.
|
|
Experimental group (%, m/n) |
Control group (%, m/n) |
|
Rate of freedom from major adverse events within 30 days |
97.5% (79/81) |
97.0% (65/67) |